In recent years, breakthroughs in neurotechnology have offered new hope for individuals living with paralysis. One particularly inspiring development is the case of a paralyzed man who successfully controlled a robotic arm through direct brain-computer interfacing. This milestone not only symbolizes a triumph of human spirit and scientific ingenuity but also opens pathways toward more accessible and intuitive assistive devices. In this in-depth article, we will explore the story behind this achievement, delve into the underlying technology, examine its benefits and challenges, and envision future prospects for persons with motor disabilities.
Imagine waking up one day unable to move your limbs, restricted not by choice but by injury or illness. For millions worldwide living with paralysis, everyday tasks—grasping a cup, feeding oneself, typing an email—become monumental obstacles. Traditional rehabilitation and assistive devices can restore some degree of independence, yet most remain externally controlled, often cumbersome, and considerably limited in dexterity.
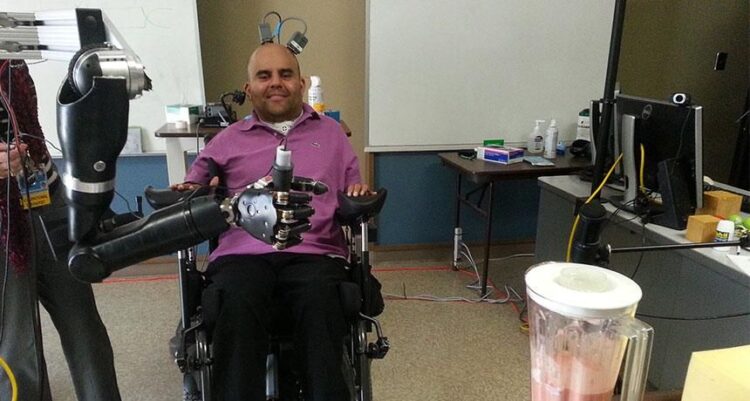
Recently, researchers collaborated with a paralyzed volunteer to bypass these limitations. By implanting a sensor array into the motor cortex—the brain’s command center for voluntary movement—the participant could manipulate a multi-jointed robotic arm simply by thinking about the movements. This seamless communication channel between mind and machine marks a paradigm shift: moving from indirect, muscle-dependent controls to fluid, thought-driven actions.
This article unpacks the journey from concept to realization, examining the lifelike control of the robotic limb, the neuroscience powering the interface, and the broader implications for rehabilitation, medicine, and society.
Case Study: A Breakthrough Moment
In a controlled laboratory setting at a leading neuroengineering institute, a 35‑year‑old man who had been tetraplegic for three years participated in a clinical trial. Following a cervical spinal cord injury, he lost voluntary control over his arms and hands. Traditional methods—sip-and-puff devices, head-mounted joysticks—allowed only rudimentary navigation of a wheelchair and limited environmental interaction.
After receiving ethical clearance and informed consent, surgeons implanted a 96‑channel microelectrode array into the primary motor cortex. Over the course of weeks, researchers trained machine‑learning algorithms to decode his neural firing patterns. Finally, in a landmark session, the participant:
A. Imagined gripping a virtual object
B. Watched the robotic arm respond in real time
C. Adapted his thoughts to refine movement accuracy
The result was awe-inspiring: he successfully reached out, grasped a small ceramic mug, lifted it, and brought it toward his mouth, all through neural intent alone. This achievement demonstrated not only the viability of direct brain control but also the user’s ability to learn and optimize mind‑machine communication.
Understanding Brain-Computer Interfaces (BCIs)
Brain-computer interfaces bridge the gap between neural activity and external devices. By translating electrical signals from neuronal networks into digital commands, BCIs enable direct control of prosthetics, computers, or even smart home systems.
Key functions of BCIs include:
A. Signal Acquisition: Capturing neural activity via invasive (microelectrode arrays) or non-invasive (EEG, fNIRS) methods.
B. Signal Processing: Filtering and amplifying raw data to isolate meaningful patterns related to specific intentions.
C. Decoding Algorithms: Employing neural networks or statistical models to map signal features onto control commands.
D. Device Actuation: Executing the decoded commands to move motors, actuators, or other output mechanisms.
While non‑invasive BCIs avoid surgery, they often sacrifice resolution and speed. Invasive approaches provide high-fidelity signals at the cost of surgical risk and long‑term biocompatibility concerns. The paralyzed man’s case underscores the power of invasive microelectrode implants, which can detect action potentials from individual neurons, enabling precise, multi-degree‑of‑freedom control.
How the Robotic Arm System Works
The integrated system connecting brain to limb comprises several stages:
-
Neural Signal Capture
-
A microelectrode array placed on the motor cortex records action potentials.
-
-
Signal Transmission
-
Neural data travel via a percutaneous connector or telemetric wireless link to an external processing unit.
-
-
Real-Time Decoding
-
Machine‑learning algorithms process streams of spikes, translating them into joint angle and velocity commands.
-
-
Motion Control
-
The decoded signals drive servo motors in the robotic arm, orchestrating coordinated movements of shoulder, elbow, wrist, and fingers.
-
-
Sensory Feedback (Emerging)
-
Some systems integrate tactile or proprioceptive feedback via sensory substitution, allowing users to “feel” pressure or position through vibration or microstimulation.
-
By iteratively calibrating decoding algorithms, researchers maximize accuracy. Each session involves the participant imagining specific movements—like wrist rotation or finger flexion—while the system learns corresponding neural signatures. Over time, the participant internalizes these mental strategies, improving speed and fluidity.
Key Components of the Technology
A successful neural-controlled prosthetic setup hinges on state‑of‑the‑art hardware and software:
A. Microelectrode Arrays
-
Hundreds of platinum‑tipped electrodes, often on a silicon backing, that penetrate cortical tissue to capture single-unit or multi-unit activity.
B. Signal Amplifiers -
Low-noise amplifiers that boost microvolt‑level neural signals without introducing artifact.
C. Wireless Telemetry Systems -
Battery‑powered transmitters that reduce infection risks associated with transcutaneous connectors, enhancing mobility and comfort.
D. Decoding Software -
Adaptive algorithms—Kalman filters, support vector machines, deep neural networks—that convert neural features into kinematic commands.
E. Robotic Prosthesis -
Lightweight, multi-degree‑of‑freedom arms with brushless DC motors, high-resolution encoders, and compliant joints for safe human interaction.
Each component demands rigorous validation. For example, microelectrode longevity is a major concern: tissue reactions can degrade signal quality over months. Ongoing research focuses on biocompatible coatings and flexible substrates to extend device lifespans.
Clinical and Ethical Considerations
Deploying invasive BCIs in human subjects requires scrupulous attention to ethics and safety:
A. Informed Consent
-
Participants must understand surgical risks, potential complications, and realistic outcomes.
B. Risk-Benefit Analysis -
Institutional review boards evaluate whether experimental gains justify risks like hemorrhage, infection, or device failure.
C. Long-Term Follow-Up -
Regular monitoring for electrode migration, tissue encapsulation, and neurological side effects.
D. Data Privacy -
Neural data are intensely personal; robust encryption and strict access controls are mandatory to prevent misuse.
E. User Autonomy -
Systems must empower users, not constrain them, preserving agency over device activation and deactivation.
Ethicists also debate scenarios where BCI-enabled prosthetics might be extended beyond medical necessity into performance enhancement—raising questions about fairness, access, and societal impacts.
Real-World Applications and Benefits
Translating laboratory success to everyday life involves addressing practical needs and optimizing user experience. Benefits include:
A. Enhanced Independence
-
Users can perform self-care activities—eating, grooming, and manipulating objects—without caregiver assistance.
B. Improved Quality of Life -
Greater autonomy fosters psychological well-being, reducing depression and enhancing social engagement.
C. Expanded Employment Opportunities -
Neural control of computers and prosthetics enables participation in a wider array of jobs and remote work.
D. Adaptive Rehabilitation -
BCIs can complement physical therapy by providing real‑time performance metrics and gamified training.
E. Research in Neuroplasticity -
Continuous BCI use may promote cortical reorganization, potentially aiding partial recovery of native motor function.
Case reports indicate that as users gain proficiency, task completion times decrease dramatically, and error rates drop below levels achievable with conventional assistive technologies.
Technical and Regulatory Challenges
Despite promising progress, several hurdles remain before widespread clinical adoption:
-
Durability of Implants
-
Chronic inflammation and glial scarring can impair signal transduction over time.
-
-
Safety of Wireless Systems
-
Power consumption, heat dissipation, and electromagnetic interference must be minimized.
-
-
Cost and Accessibility
-
Current BCI systems can cost upwards of $100,000, placing them out of reach for most patients without substantial insurance coverage or subsidies.
-
-
Regulatory Approval
-
Agencies like the U.S. FDA or the European CE require extensive safety and efficacy data from multi‑site trials.
-
-
Standardization
-
Lack of unified protocols for signal processing, device interoperability, and outcome metrics complicates cross‑center collaboration.
-
Addressing these challenges demands multidisciplinary collaboration among neuroscientists, engineers, clinicians, ethicists, and policymakers.
Advances on the Horizon
Ongoing research promises to refine and expand neural-prosthetic capabilities:
A. Flexible and Biodegradable Electrodes
-
Next-generation materials that conform to brain tissue and gradually dissolve, reducing long-term risks.
B. High‑Density Neural Interfaces -
Arrays with thousands of microelectrodes offering finer spatial resolution and richer control.
C. Closed‑Loop Feedback Systems -
Integrating sensory input via cortical microstimulation to provide tactile feedback, enabling nuanced grip force and slip prevention.
D. Non‑Invasive Optical BCIs -
Techniques like functional ultrasound imaging that may achieve near-invasive resolution without surgery.
E. Machine Learning Advances -
Deep reinforcement learning algorithms that adapt to evolving neural patterns, reducing calibration time and improving robustness.
Such innovations could eventually lead to fully implantable, armband‑style BCIs or even neural dust—tiny sensors dispersed across the cortex—ushering in an era of seamless mind‑machine symbiosis.
Conclusion
The story of a paralyzed man regaining hand-like control through a robotic arm is more than a scientific milestone—it is a testament to human ingenuity and hope. While challenges in safety, cost, and regulation persist, the trajectory of neurotechnology points toward a future where paralysis need not define limitation. As research accelerates, we edge closer to a world in which the boundary between mind and machine dissolves, enabling people with motor impairments to interact with their environment as naturally as we do. This progress offers not only independence and mobility but also dignity and the profound satisfaction of reclaiming one’s agency.